Aki Saito, Wataru Igarashi, Hiroyuki Furukawa, Teiko Yamada, Shigefumi Kuwahara, Hiromasa Kiyota, Nat. Prod. Commun., 10 (4), 645-648 (2015).
200. "Facile Synthesis of the Cyclohexane Fragment of Enacyloxins, a Series of Antibiotics Isolated from Frateruria sp. W-315
Aki Saito, Wataru Igarashi, Hiroyuki Furukawa, Teiko Yamada, Shigefumi Kuwahara, Hiromasa Kiyota, Biosci. Biotechnol. Biochem., 78 (5), 766-769 (2014).
156. "Synthesis of C16'-C23' Fragments of Enacyloxins, A Series of Antibiotics from Frateuria sp. W-315"
Wataru Igarashi, Hiroaki Hoshikawa, Hiroyuki Furukawa, Teiko Yamada, Shigefumi Kuwahara, Hiromasa Kiyota, Heterocycl. Commun., 17(1-2), 7-9 (2011).
155. "Synthesis of a C9'-C15' Fragment of Enacyloxins, A Series of Antibiotics from Frateuria sp. W-315"
Hiroyuki Furukawa, Hiroaki Hoshikawa, Wataru Igarashi, Manabu Yaosaka, Teiko Yamada, Shigefumi Kuwahara, Hiromasa Kiyota, Heterocycl. Commun., 17(1-2), 3-5 (2011).
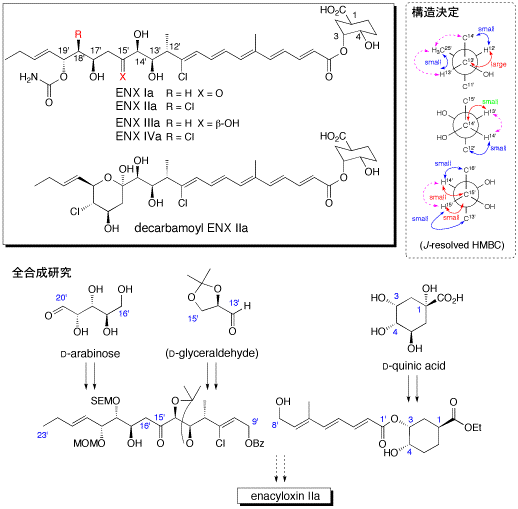
109. "Setereochemistry of Enacyloxins 4. Completion of Structure Assignment with (13'R, 14'R, 15'S)-Configuration of Enacyloxins, a Series of Antibiotics from Frateuria sp. W-315"
Hiroyuki Furukawa, H. Kiyota, T. Yamada, Manabu Yaosaka, Ryo Takeuchi, Toshihiko Watanabe, S. Kuwahara, Chem. Biodivers., 4, 1601-1604 (2007).
73. "Intramolecular Migration of Long Chain Acyl Group of Enacyloxin IIa Methyl Ester"
T. Sugiyama, H. Kiyota, T. Watanabe, T. Oritani, Nat. Prod. Res., 19(6), 581-584 (2005).
48. "Stereochemistry of Enacyloxins 3. (12'S, 17'R, 18'S, 19'R)-Absolute Configuration of the Polyene Part of Enacyloxin IIa (ENX IIa)"
R. Takeuchi, H. Kiyota, M. Yaosaka, T. Watanabe, the late T. Sugiyama, T. Oritani, J. Chem. Soc., Perkin Trans. 1, 2001(20), 2676-2681.
44. "Stereochemistry of Enacyloxins 2. Structure Elucidation of Decarbamoyl Enacyloxin IIa and IVa, New Members of Enacyloxin Antibiotics from Frateuria sp. W-315"
T. Watanabe, H. Kiyota, R. Takeuchi, K. Enari, T. Oritani, Heterocycl. Commun., 7(4), 313-316 (2001).
43. "Stereochemistry of Enacyloxins 1. Absolute Configurtion of the Cyclohexane Part of Enacyloxins, a Series of Antibiotics from Frateuria sp. W-315"
T. Fujimori, O. Nakayama, H. Kiyota, Y. Kamijima, T. Watanabe, T. Oritani, Heterocycl. Commun., 7(4), 327-330 (2001).