Antibiotics 1
Polynacins (Actins)
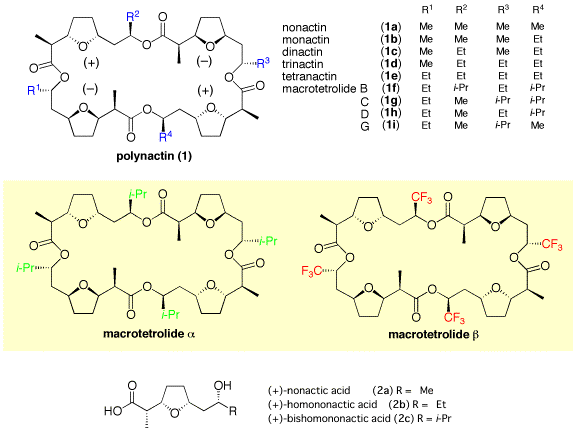
The macrotetrolide ionophore antibiotic "polynactin" family (1), which have been isolated from various Streptomyces species, are composed of both enantiomers of nonactic acid (2a), homononactic acid (2b) or bishomononactic acid (2c) arranged in an alternating order, namely, a meso-like-form. Curiosity about these structures has already led many chemists to synthesize these monomers and tetramers. A mixture of the parts (1a-1e) is used as an acaricide for fermentative production. Although there is no information about the biological activities of macrotetrolides B~G (1f~1i), the antimicrobial and antifungal activities of nonactin (1a), monactin (1b), dinactin (1c) and trinactin (1d) increase with increasing number of the ethyl substituent. To study the biological activities of bishomononactic acid (2c) and macrotetrolides B-G (1f-1i), which might be more active, designed bulky analogs "macrotetrolides alpha (i-Pr)4 & beta (CF3)4" have been prepared.
168. "Synthesis of omega-Trifluorononactic Acid, a Designed Analog of Polynactin Monomer"
K. Takai, T. Hanadate, S. Oi, T. Yamada, S. Kuwahara, H. Kiyota, Synthesis, 2011, 3741-3748.
163. "Biotransformation of a Biosynthetic Intermediate Mimic of Nonactin by Streptomyces griseus"
T. Hanadate, K. Takai, T. Yamada, S. Kuwahara, H. Kiyota, Hetrocycl. Commun., 17(3-4), in press (2011).
160. "Synthesis of Macrotetraolide alpha, a Designed Polynactin Analog Composed of Bishomononactic Acid"
K. Takai, T. Hanadate, T. Yamada, S. Kuwahara, H. Kiyota, Tetrahedron, 67(37), 7066-7072 (2011).
45. "Synthesis of Mactotetrolide alfa, a Designed Polynactin Analog Composed of (+)- and (-)-Bishomononactic Acids"
T. Hanadate, H. Kiyota and T. Oritani, Biosci. Biotech. Biochem., 65(9), 2118-2120 (2001).
28. "Synthesis of (+-)-Methyl Bishomononactate via Iodoetherification"
T. Hanadate, H. Kiyota and T. Oritani, Biosci. Biotech. Biochem., 64(8), 1671-1674 (2000).
Synthesis route 2 Synthesis route 3
14. "Synthesis of (+)-Homononactic Acid via Iodoetherification"
H. Kiyota, M. Abe, Y. Ono and T. Oritani, Synlett, 1997, 1093-1095.
Synthesis route 1
12. "Synthesis of (±)-Homononactic Acid by Using cis-Selective Iodoetherification"
M. Abe, H. Kiyota, M. Adachi and T. Oritani, Synlett, 1996, 777-778.
Pamamycins
Pamamycins are a series of antibiotic macrodiolides isolated from various Streptomyces species. Among them, pamamycin-607 (1) exhibited aerial mycelium-inducing activity for an aerial mycelium-negative mutant strain and showed antibiotic activity against Gram positive bacteria and fungi. 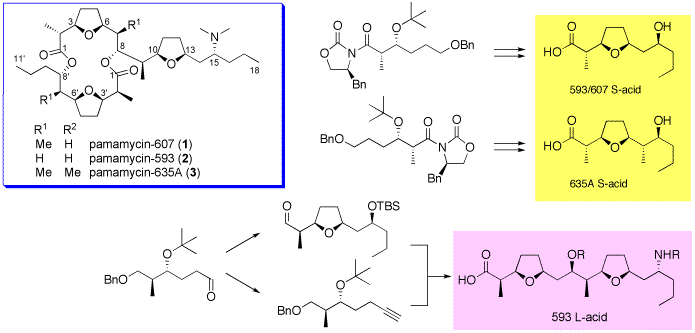
125. "Synthesis of L-Acid (C1-C18) Fragment of Pamamycin-593 and De-N-methylpamamycin-579"
Ayako Miura, Shin-ya Takigawa, Yukito Furuya, Yuhsuke Yokoo, S. Kuwahara, H. Kioyta, Eur. J. Org. Chem., 4955-4962 (2008).
69. "Synthesis of the Southern Fragment of Pamamycin-635A"
Ayako Miura, H. Kioyta and S. Kuwahara, Tetrahedron, 61, 1061-1067 (2005).
49. "Synthesis of the Southern and the Eastern Parts of Pamamycin-607, an Aerial Mycelium-Inducing Substance from Streptomyces alboniger"
Y. Furuya, H. Kiyota and T. Oritani, Biosci. Biotech. Biochem., 65(12), 2630-2637 (2001).
35. "Synthesis of the Southern Part of Pamamycin-607, an Aerial Mycelium-Inducing Substance from Streptomyces alboniger"
Y. Furuya, H. Kiyota and T. Oritani, Heterocycl. Commun., 6 (5), 427-430 (2000).
Glutarimides
Cycloheximide is a famous antibiotics because of its strong and broad antimicrobial spectra, however, the toxicity limits its use. Actiketal (RK-441S) was isolated from the culture extracts of Streptomyces pulveraceus subsp. epiderstagenes as a new spiroacetal-type glutarimide antibiotic. This compound inhibited the EGF-induced DNA formation in murine epithelial cell (100% at 1 μM) and the Con A-induced blast formation in spleen cell (100% at 20 nM) Actiketal is expected to be a new anticancer agent and an immnosuppressant because of its low cytotoxicity.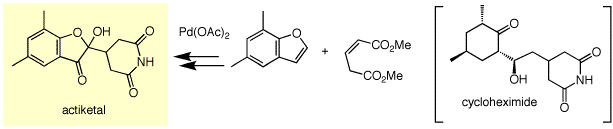
93. "Synthetic Studies on Heterocyclic Antibiotics Containing Nitrogen Atoms" REVIEW
H. Kiyota, in "Topic in Heterocyclic Chemistry 06: Bioactive Heterocyles 1", ed. S. Eguchi, Chapter 6, Springer 2006, pp 181-214.
38. "Synthesis of Actiketal, a Unique Benzofuran Type Glutarimide Antibiotic from Streptomyces pulveraceus"
H. Kiyota, Y. Shimizu and T. Oritani, J. Pesticide Sci., 25 (1), 93-95 (2001).
27. "Synthesis of Actiketal, a Glutarimide Antibiotic"
H. Kiyota, Y. Shimizu and T. Oritani, Tetrahedron Lett., 41, 5887-5890 (2000).
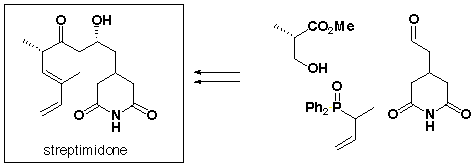
177. "Structure-activity Relationship of 9-Methylstreptimidone that Induces Selective Apoptosis in Adult T-cell Leukemia Cells"
M. Takeiri, E. Ota, S. Nishiyama, H. Kiyota, K. Umezawa, Oncol. Res., 20 (1), 7-14 (2012).
32. "Synthesis and Antifungal Activity of the Four Stereoisomers of Streptimidone, a Glutarimide Antibiotic from Streptomyces rimosus forma paromomycinus"
H. Kondo, T. Oritani and H. Kiyota, Eur. J. Org. Chem., 2000, 3459-3462.
Spirofungins
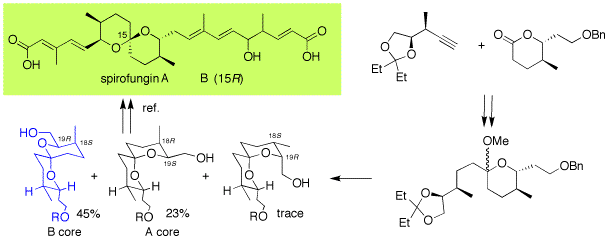
Spirofungins A and B were isolated as a 4:1 mixture from Streptomyces violaceusniger Tu 4113 as new polyketide-spiroacetal related to reveromycins.
 Co-operation:H.-P. Fiedler, (Tubingen, Deutche), L. C. Dias (Campinas, Brazil)
Co-operation:H.-P. Fiedler, (Tubingen, Deutche), L. C. Dias (Campinas, Brazil)
207. "Synthesis of the Spiroacetal Fragments of Spifofungins A and B, Antibiotics Isolated from Streptomyces violaceusniger Tu 4113"
H. Sakauchi, E. Higashi, Y. Shimizu, M. Kojima, Y. Asamitsu, S. Kuwahara, M. Izumi, H. Kiyota, Heterocycl. Commun., 21 (6), 337-343 (2015).
85. "Spirofungins A and B: a Reassignment of Kiyota's Spiroacetals"
L. G. de Oliveira, L. C. Dias, Hiroyuki Sakauchi, H. Kiyota, Tetrahedron Lett., 47, 2413-2418 (2006).
25. "Synthesis of the Spiroacetal Parts of Spirofungin A and B"
Y. Shimizu, H. Kiyota and T. Oritani, Tetrahedron Lett., 41, 3141-3144 (2000).




