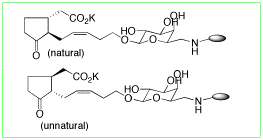
Methyl jasmonate (MJA), at first isolated as an important constituents of jasmine absolute, is recently recognized as one of the plant hormones.
MJA and jasmonic acid (JA) sustain many plant actions such as plant growth inhibition, senescence promotion, abscission, tuber formation, stress response etc. Recent studies suggested taht really active compound is its 7-epimer, epijasmonic acid (epiJA). Actually, methyl epijasmonate is epimerized to MJA as 20:1 mixtyure at equilibrium.
We have prepared some MJA analogs with 7-substituents to fix the epimerizaion.
70. Synthesis and Biological Evaluation of Abscisic Acid, Jasmonic Acid and their Analogs"[REVIEW]
H. Kiyota, T.i Oritani, S. Kuwahara, "New Discoveries in Agrochemiscals" ACS symposium series No. 892; J. M. Clark, H. Ohkawa eds., pp 246-254 (2005).
15. "Synthesis and Biological Activities of 3,7-and 4,5-Didehydrojasmonates"
H. Kiyota, Y. Yoneta and T. Oritani, Phytochemistry, 46, 983-986 (1997).
2. Methyl jasmonate analogs 2
12-Hydroxyepijasmonic acid (tuberonic acid, TA, 4) was isolated as potato tuber-forming substance. On the other hand, its biosynthetic precursor epiJA, and MJA also showed this activity. We prepared 12,12,12-trifluoro-MJA 5 to know the necessity of the OH group of TA, and found that this OH group is not essential for the activity.
10. "Synthesis and Potato Tuber-Inducing Activity of Methyl 5',5',5'-Trifluorojasmonate"
H. Kiyota, M. Saitoh, T. Oritani and T. Yoshihara, Phytochemistry, 42, 1259-1262 (1996).
39. "(±)-Methyl 11-Fluorojasmonate as a Designed Antimetabolite of Methyl Jasmonate: Synthesis and Plant Growth Regulatory Activity"
H. Kiyota, T. Koike, E. Higashi, Y. Satoh and T. Oritani, J. Pesticide Sci., 26, 96-99.
3. Synthesis of methyl tuberonate (4)
Scheme
23. "Synthesis of Methyl Tuberonate, a Potato Tuber-forming Substance"
H. Kiyota, D. Nakashima, T. Oritani, Biosci. Biotech. Biochem., 63 (12), 2110-2117 (1999).
4. Preparation of enantiopure methyl jasmonates
A facile method to supply natural and unnnatural enantiomers of methyl jasmonate and its 4,5-didehydro derivative by lipase-catalyzed resolution of racemic methyl 7-epi-cucurbate was developed.
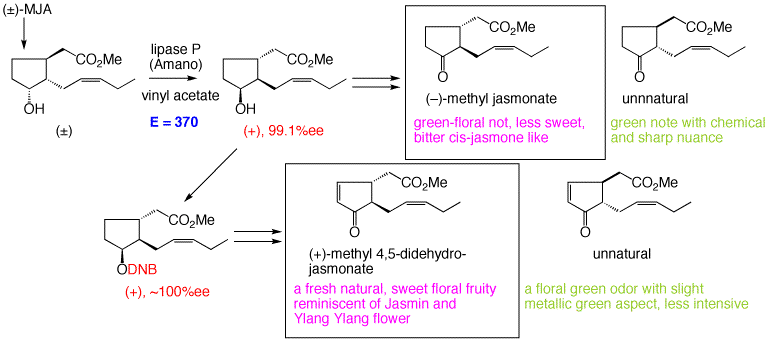
41. "Lipase-catalyzed preparation of both enantiomers of methyl jasmonate"
H. Kiyota, E. Higashi, T. Koike, T. Oritani, Tetrahedron: Asymmetry, 12, 1035-1038 (2001).
129. "Enzymatic Resolution of (±)-2-Exo-7-syn-7-(1-propynyl)norbornan-2-ol, a Key Synthetic Intermediate for Jasmonoids"
Hiromasa Kiyota, Daisuke Nakashima, Shigefumi Kuwahara, Takayuki Oritani, Zeitschrift fuer Narurforshung, 63b (12), 1441-1442 (2008).