Odor 1
1. Enzymatic optical resolution of methyl jasmonate (MJA)
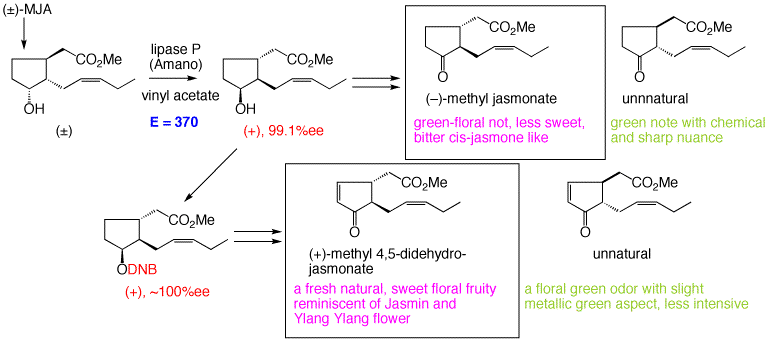
(-)-Methyl jasmonate [(-)-1] isolated at first from jasmine absolute as a jasmine note have been found to play important roles in plant growth regulation, such as plant growth promotion/inhibition, potato tuber formation, signal transduction etc. Recent development of industrial production of (±)-methyl jasmonate [(±)-1] from Nippon Zeon Co., Ltd. made it possible to use 1 practically. We developed a facile method to supply natural and unnnatural enantiomers of methyl jasmonate by lipase-catalyzed resolution of racemic methyl 7-epi-cucurbate (overall 40% yield!).
Absoluted stereochemistry of methyl 4,5-dehydrojasmonate, a minor constituent of jasmine absolute, was determined and its odor was evaluated.
95. "Enzymatic Resolution of (±)-2-Exo-7-syn-7-(1-propynyl)norbornan-2-ol, a Key Synthetic Intermediate for Jasmonoids"
Hiromasa Kiyota, Daisuke Nakashima, Shigefumi Kuwahara, Takayuki Oritani, Zeitschrift fuer Narurforshung, 63b (12), 1441-1442 (2008).
41. "Lipase-catalyzed Preparation of Both Enantiomers of Methyl Jasmonate"
H.Kiyota, E. Higashi, T. Koike, T. Oritani, Tetrahedron: Asymmetry, 12, 1035-1038 (2001).
91. "Synthesis and odor Description of Both Enantiomers of Methyl 4,5-Didehydrojasmonate"
Y. Asamitsu, Y. Nakamura, M. Ueda, S. Kuwahara, H.Kiyota, Chem. Biodivers., 3, 654-659 (2006).
2. Enzymatic optical resolution of lavandulol
Both enantiomers of lavandulol, an important constituent of lavender oil, were prepared by enzymatic optical resolution, and the odor quality of the single antipodes was evaulated.
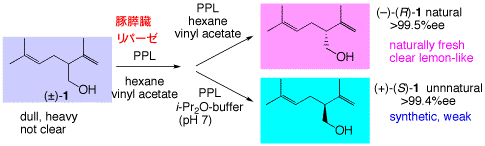
77. "Enzymatic Resolution and Odour Description of Both Enantiomers of Lavandulol, a Fragrance of Lavender Oil"
Hiroyuki Sakauchi, H. Kiyota, Shi-ya Takigawa, T. Oritani, S. Kuwahara Chemistry & Biodiversity, 2, 1183-1186 (2005).
3. Optical resolution of irones
The principal constituents of iris oil, (-)-cis-alpha-irone and (-)-cis-gamma-irone, and their enantiomers, were synthesized, and the odor quality was evaluated.
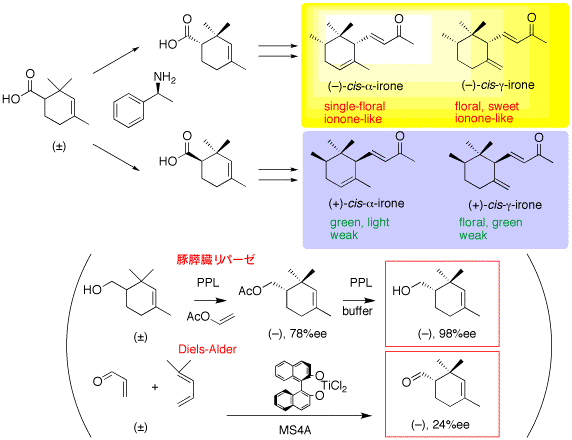
33. "Synthesis of both enantiomers of cis-alfa-irone and cis-gamma-irone, principal constituents of Irish oil, via optical resolution of (+-)-2,2,4-trimethyl-3-cyclohexene-1-carboxylic acid"
T. Inoue, H. Kiyota, T. Oritani, Tetrahedron: Asymmetry, 11, 3807-3818 (2000).
4. Tricarbocylic compound
Chlorosulfonic acid catalyzed cyclization reaction of beta-monocyclonerolidol and beta-monocyclofarnesol afforded tricarbocyclic compound with a creamy odor.
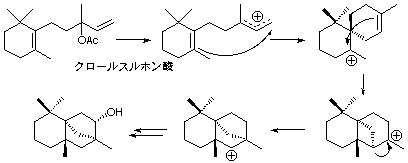
13. "Stereochemistry of a Unique Tricarbocyclic Compound Prepared by Superacid-Catalyzed Cynclization"
H. Tanimoto, H. Kiyota, T. Oritani and K. Matsumoto, Synlett, 1997, 121-122.


