Publication
"Toward the Synthesis of Paspaline-Type Indole-Terpenes: Stereoselective Construction of Core Scaffold with Contiguous Asymmetric Quaternary Carbon Centers"
Ichiro Hayakawa*, Naochika Matsumaru, and Akira Sakakura*
J. Org. Chem. 2021, ASAP
DOI: 10.1021/acs.joc.1c01193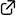
The core scaffold of paspaline-type indole-terpenes was synthesized by using the House–Meinwald rearrangement as a key step. Rearrangement of the epoxide methyl group in the precursor with methylaluminum bis(4-bromo-2,6-di- tert-butylphenoxide) as a Lewis acid proceeded smoothly to construct contiguous asymmetric quaternary carbon centers by a 1,2-chirality transfer.
(2021.7.7)
"Toward the Synthesis of Paspaline-Type Indole-Terpenes: Stereoselective Construction of Core Scaffold with Contiguous Asymmetric Quaternary Carbon Centers"
Ichiro Hayakawa*, Naochika Matsumaru, and Akira Sakakura*
J. Org. Chem. 2021, ASAP
DOI: 10.1021/acs.joc.1c01193
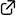

The core scaffold of paspaline-type indole-terpenes was synthesized by using the House–Meinwald rearrangement as a key step. Rearrangement of the epoxide methyl group in the precursor with methylaluminum bis(4-bromo-2,6-di- tert-butylphenoxide) as a Lewis acid proceeded smoothly to construct contiguous asymmetric quaternary carbon centers by a 1,2-chirality transfer.
(2021.7.7)
Publication
"Strain-Release Difunctionalization of C–C σ- and π-bonds of an Organoboron Ate-Complex through 1,2-Metallate Rearrangement"
Haruki Mizoguchi,* Akira Sakakura*
Chem. Lett. 2021, 50, 792–799.
DOI: 10.1246/cl.200926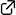
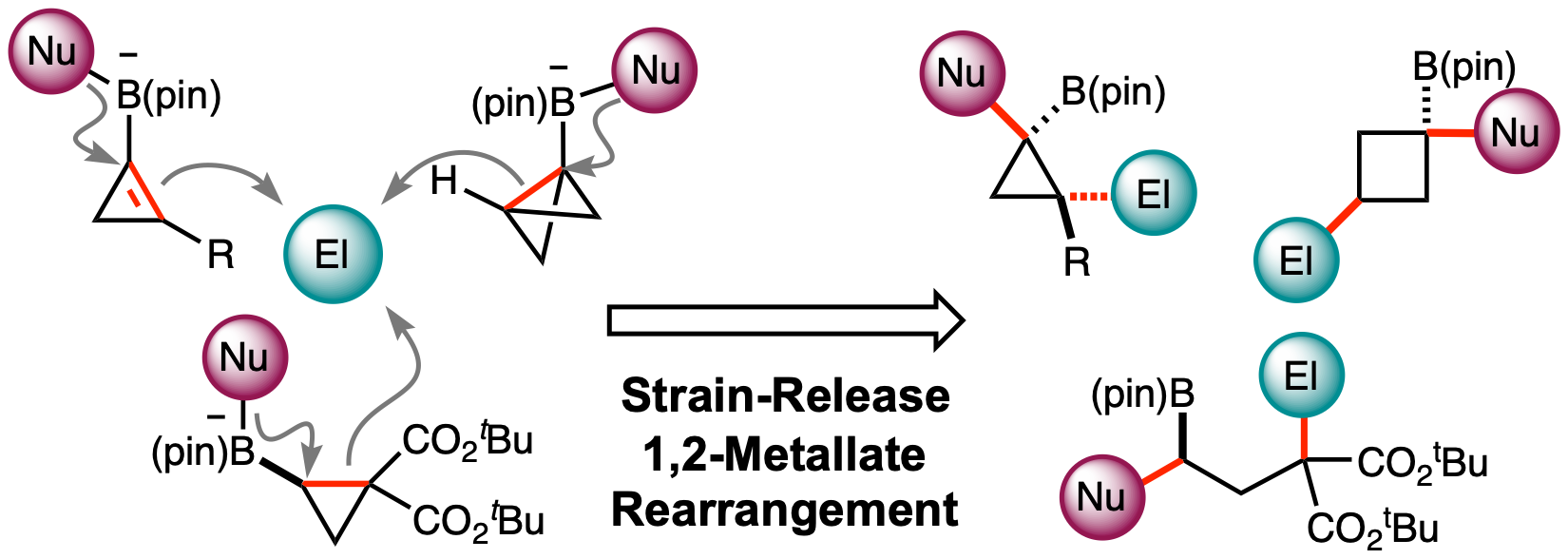
This highlight review describes the recent development of an electrophile-triggered 1,2-metallate rearrangement of organoboronic ester ate-complex, which proceeds through 1,2-difunctionalization of carbon–carbon σ- and π-bonds, using strain energy as a driving force. Coupling reactions of small ring carbocyclic boronic esters, such as cyclopropyl-, bicyclo[1.1.0]butyl-, and cyclopropenyl-boronic ester, are summarized along with the proposed reaction mechanisms and representative examples.
"Strain-Release Difunctionalization of C–C σ- and π-bonds of an Organoboron Ate-Complex through 1,2-Metallate Rearrangement"
Haruki Mizoguchi,* Akira Sakakura*
Chem. Lett. 2021, 50, 792–799.
DOI: 10.1246/cl.200926
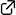

This highlight review describes the recent development of an electrophile-triggered 1,2-metallate rearrangement of organoboronic ester ate-complex, which proceeds through 1,2-difunctionalization of carbon–carbon σ- and π-bonds, using strain energy as a driving force. Coupling reactions of small ring carbocyclic boronic esters, such as cyclopropyl-, bicyclo[1.1.0]butyl-, and cyclopropenyl-boronic ester, are summarized along with the proposed reaction mechanisms and representative examples.
Publication
"Synthesis of functionalized cyclopropylboronic esters based on a 1,2-metallate rearrangement of cyclopropenylboronate"
Haruki Mizoguchi,* Masaya Seriu, Akira Sakakura*
Chem. Commun., Advance Article
DOI: 10.1039/d0cc07134j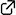
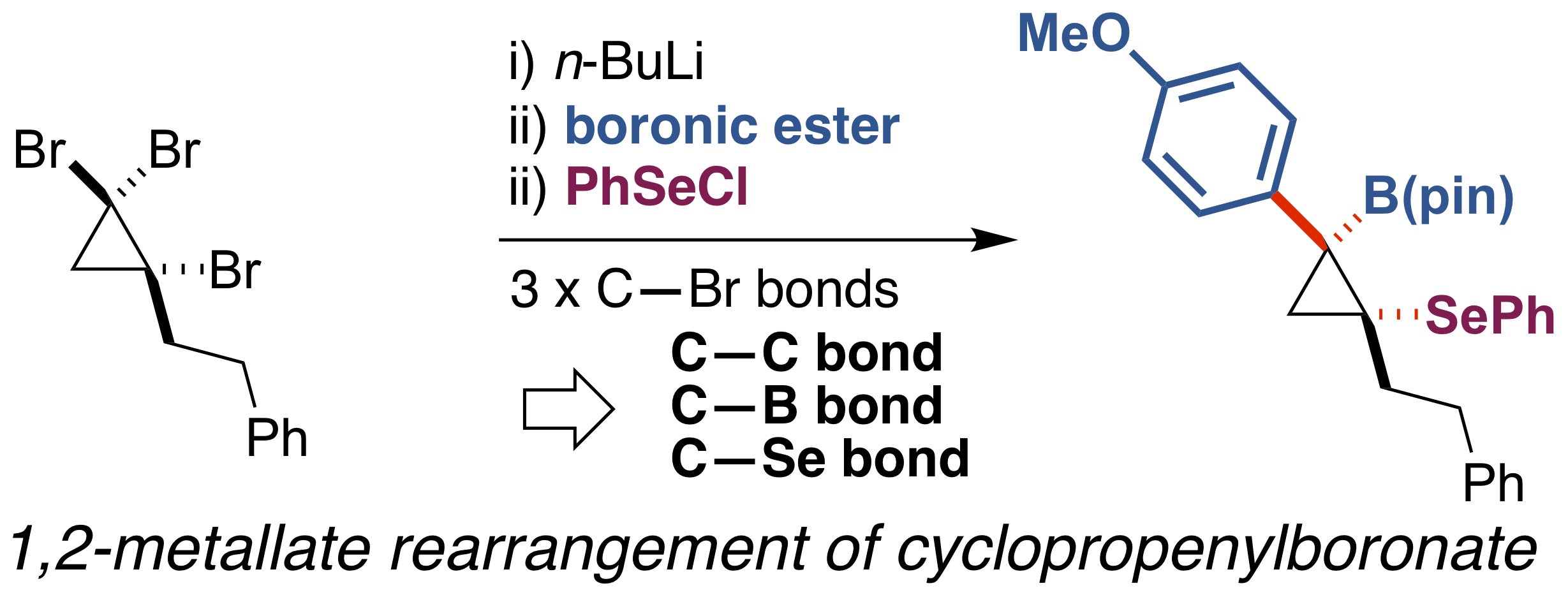
A procedure converting tribromocyclopropane to densely functionalized β-selenocyclopropylboronic ester using the 1,2-metallate rearrangement of a boron ate-complex has been developed. Treatment of an in situ-generated cyclopropenylboronic ester ate-complex with phenylselenenyl chloride triggered stereospecific rearrangement to produce functionalized cyclopropanes. DFT calculations for 1,2-metallate rearrangement suggested that the reaction proceeds through a seleniranium intermediate.
(2020.11.8)
"Synthesis of functionalized cyclopropylboronic esters based on a 1,2-metallate rearrangement of cyclopropenylboronate"
Haruki Mizoguchi,* Masaya Seriu, Akira Sakakura*
Chem. Commun., Advance Article
DOI: 10.1039/d0cc07134j
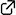

A procedure converting tribromocyclopropane to densely functionalized β-selenocyclopropylboronic ester using the 1,2-metallate rearrangement of a boron ate-complex has been developed. Treatment of an in situ-generated cyclopropenylboronic ester ate-complex with phenylselenenyl chloride triggered stereospecific rearrangement to produce functionalized cyclopropanes. DFT calculations for 1,2-metallate rearrangement suggested that the reaction proceeds through a seleniranium intermediate.
(2020.11.8)
Publication
"Kinetic Resolution of α-Nitrolactones by Catalytic Asymmetric Hydrolysis or Ester–Amide Exchange Reaction"
Ryota Nakao, Yudai Fujii, Ichiro Hayakawa, Haruki Mizoguchi, Akira Sakakura*
Synlett, eFirst
DOI: 10.1055/s-0040-1707303
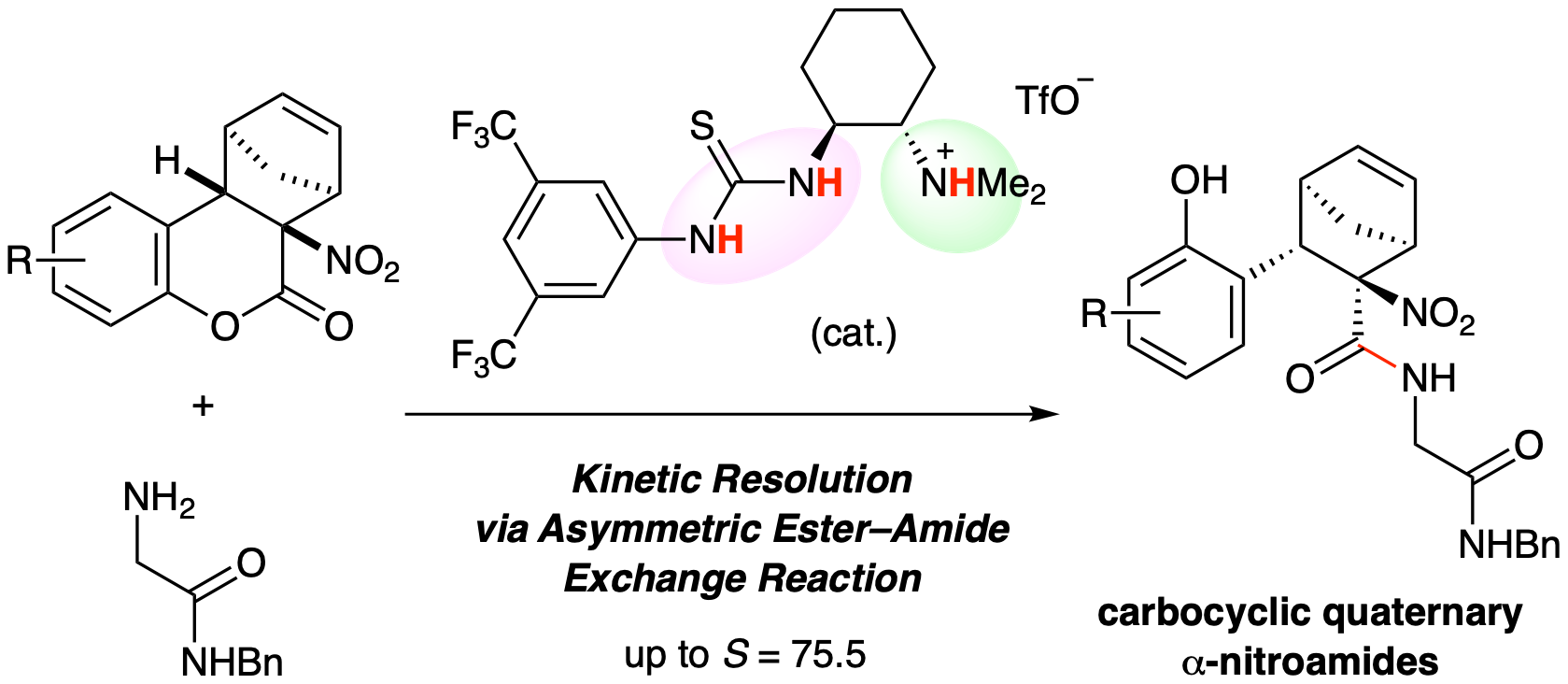
C1-Symmetric chiral ammonium salt catalysts induced a kinetic resolution of racemic α-nitrolactones through an asymmetric ester–amide exchange reaction. The corresponding amides were obtained with high enantioselectivities and high S (= kfast/kslow) values. This reaction system is a useful approach for obtaining carbocyclic quaternary α-nitroamides as chiral building blocks.
(2020.10.8)
"Kinetic Resolution of α-Nitrolactones by Catalytic Asymmetric Hydrolysis or Ester–Amide Exchange Reaction"
Ryota Nakao, Yudai Fujii, Ichiro Hayakawa, Haruki Mizoguchi, Akira Sakakura*
Synlett, eFirst
DOI: 10.1055/s-0040-1707303
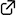

C1-Symmetric chiral ammonium salt catalysts induced a kinetic resolution of racemic α-nitrolactones through an asymmetric ester–amide exchange reaction. The corresponding amides were obtained with high enantioselectivities and high S (= kfast/kslow) values. This reaction system is a useful approach for obtaining carbocyclic quaternary α-nitroamides as chiral building blocks.
(2020.10.8)
Publication
"Enantioselective Diels–Alder Reaction of 3-Nitrocoumarins Promoted by Chiral Organoammonium Salt Catalysts"
Yudai Fujii, Ryota Nakao, Saki Sugihara, Keita Fujita, Yuya Araki, Takayuki Kudoh, Ichiro Hayakawa, Haruki Mizoguchi, Akira Sakakura*
Synlett, eFirst
DOI: 10.1055/s-0040-1707302
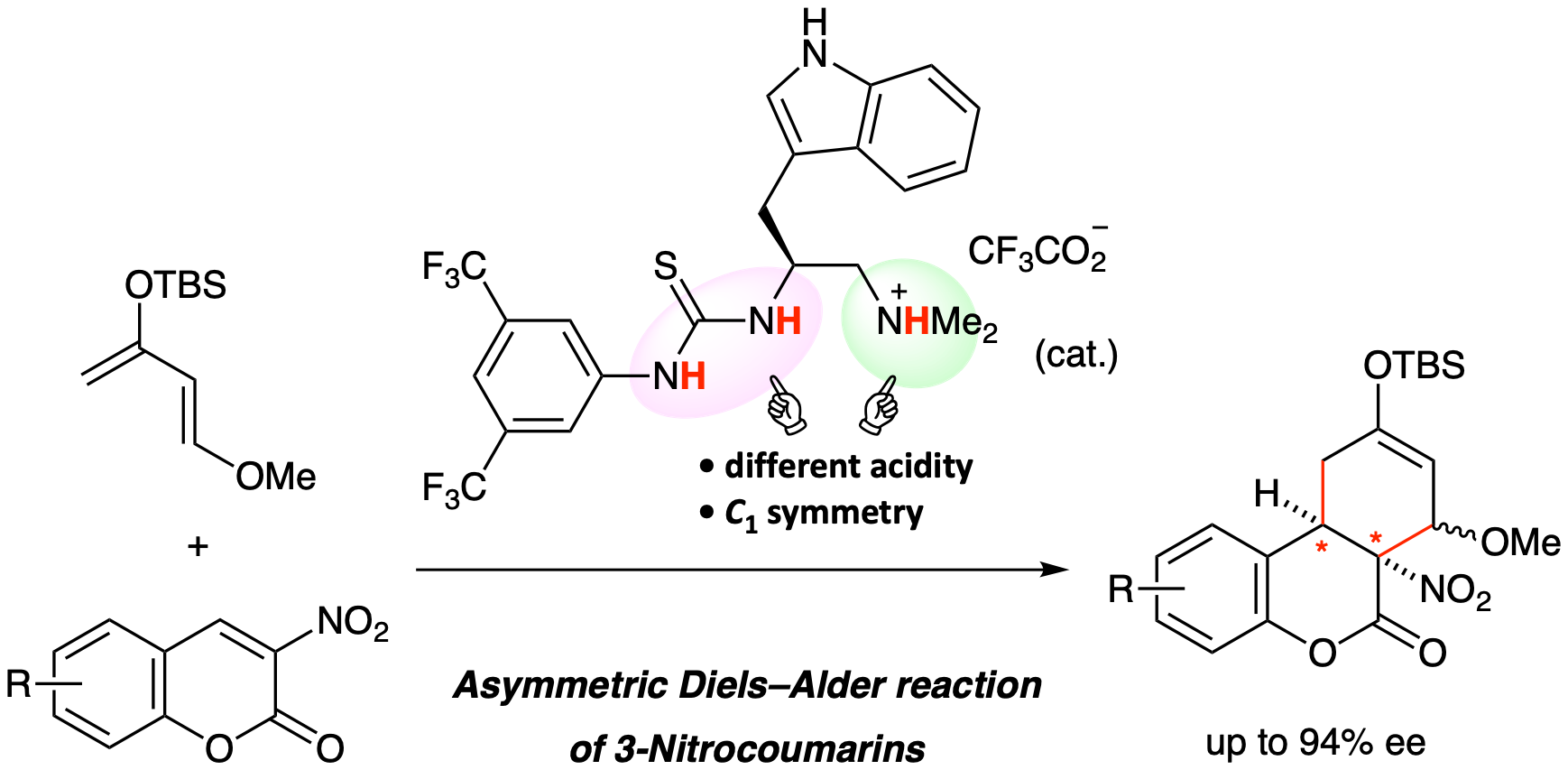
An enantioselective Diels–Alder reaction of 3-nitrocoumarins has been developed. A tryptophan-derived C1-symmetric organoammonium thiourea catalyst promoted the reaction of 3-nitrocoumarins with Danishefsky’s diene to give the corresponding adducts with good enantioselectivity (up to 94% ee). One of the resulting adducts was converted into a chiral carbocyclic quaternary β-amino alcohol.
(2020.10.7)
"Enantioselective Diels–Alder Reaction of 3-Nitrocoumarins Promoted by Chiral Organoammonium Salt Catalysts"
Yudai Fujii, Ryota Nakao, Saki Sugihara, Keita Fujita, Yuya Araki, Takayuki Kudoh, Ichiro Hayakawa, Haruki Mizoguchi, Akira Sakakura*
Synlett, eFirst
DOI: 10.1055/s-0040-1707302
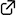

An enantioselective Diels–Alder reaction of 3-nitrocoumarins has been developed. A tryptophan-derived C1-symmetric organoammonium thiourea catalyst promoted the reaction of 3-nitrocoumarins with Danishefsky’s diene to give the corresponding adducts with good enantioselectivity (up to 94% ee). One of the resulting adducts was converted into a chiral carbocyclic quaternary β-amino alcohol.
(2020.10.7)
The academic year 2020 has just started with 9 graduate students and 9 undergraduate students.
(2020.4.1)
Publication
"Structure Optimization of Gatastatin for the Development of γ-Tubulin-Specific Inhibitor"
Kana Shintani, Haruna Ebisu, Minagi Mukaiyama, Taisei Hatanaka, Takumi Chinen, Daisuke Takao, Yoko Nagumo, Akira Sakakura, Ichiro Hayakawa,* Takeo Usui*
ACS Med. Chem. Lett. 2020, 11 (6), 1125–1129.
DOI: 10.1021/acsmedchemlett.9b00526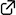
Gatastatin (O7-benzyl glaziovianin A) is a γ-tubulin-specific inhibitor that is used to investigate γ-tubulin function in cells. We have previously reported that the unsubstituted phenyl ring of the O7-benzyl group in gatastatin is important for γ-tubulin inhibition. To obtain further structural information regarding γ-tubulin inhibition, we synthesized several gatastatin derivatives containing a fixed O7-benzyl moiety. Modifications of the B-ring resulted in drastic decrease in cytotoxicity, abnormal spindle formation activity, and inhibition of microtubule (MT) nucleation. In contrast, various O6-alkylated gatastatin derivatives showed potent cytotoxicity, induced abnormal spindle formation, and inhibited MT nucleation. We had previously reported that O6-benzyl glaziovianin A is a potent α/β-tubulin inhibitor; thus, these new results suggest that the O6-position restricts affinity for α/β- and γ-tubulin. Considering that an O7-benzyl group increases specificity for γ-tubulin, more potent and specific γ-tubulin inhibitors can be generated through O6-modifications of gatastatin.
(2020.3.30)
"Structure Optimization of Gatastatin for the Development of γ-Tubulin-Specific Inhibitor"
Kana Shintani, Haruna Ebisu, Minagi Mukaiyama, Taisei Hatanaka, Takumi Chinen, Daisuke Takao, Yoko Nagumo, Akira Sakakura, Ichiro Hayakawa,* Takeo Usui*
ACS Med. Chem. Lett. 2020, 11 (6), 1125–1129.
DOI: 10.1021/acsmedchemlett.9b00526

Gatastatin (O7-benzyl glaziovianin A) is a γ-tubulin-specific inhibitor that is used to investigate γ-tubulin function in cells. We have previously reported that the unsubstituted phenyl ring of the O7-benzyl group in gatastatin is important for γ-tubulin inhibition. To obtain further structural information regarding γ-tubulin inhibition, we synthesized several gatastatin derivatives containing a fixed O7-benzyl moiety. Modifications of the B-ring resulted in drastic decrease in cytotoxicity, abnormal spindle formation activity, and inhibition of microtubule (MT) nucleation. In contrast, various O6-alkylated gatastatin derivatives showed potent cytotoxicity, induced abnormal spindle formation, and inhibited MT nucleation. We had previously reported that O6-benzyl glaziovianin A is a potent α/β-tubulin inhibitor; thus, these new results suggest that the O6-position restricts affinity for α/β- and γ-tubulin. Considering that an O7-benzyl group increases specificity for γ-tubulin, more potent and specific γ-tubulin inhibitors can be generated through O6-modifications of gatastatin.
(2020.3.30)
Publication
"Formal Total Synthesis of Manzacidin B via Sequential Diastereodivergent Henry Reaction"
Yuya Araki, Natsumi Miyoshi, Kazuki Morimoto, Takayuki Kudoh, Haruki Mizoguchi, Akira Sakakura*
J. Org. Chem. 2020, 85 (2), 798–805.
DOI: 10.1021/acs.joc.9b02811
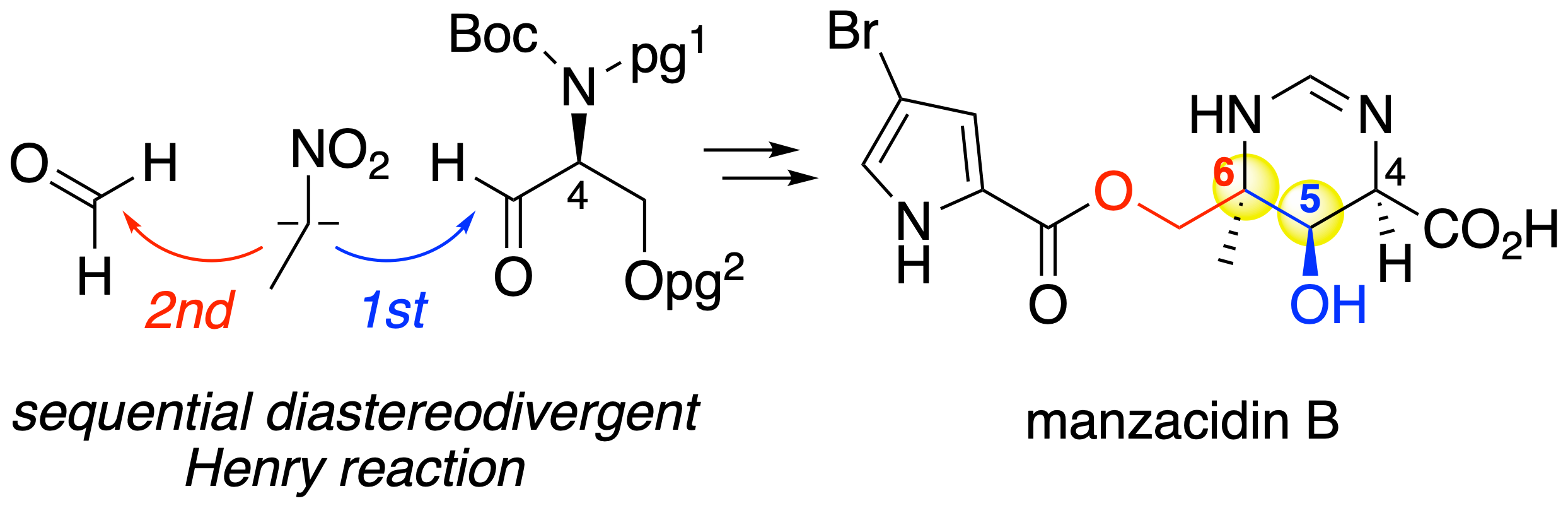
A formal total synthesis of manzacidin B is described. β,β-Disubstituted γ-hydroxy-β-aminoalcohol, the key structure of manzacidin B, is stereoselectively constructed via sequential Henry reactions. By taking advantage of noncovalent interactions, such as intramolecular hydrogen bonding and chelation, we could diastereodivergently control the stereoselectivity of the Henry reaction.
(2019.12.18)
"Formal Total Synthesis of Manzacidin B via Sequential Diastereodivergent Henry Reaction"
Yuya Araki, Natsumi Miyoshi, Kazuki Morimoto, Takayuki Kudoh, Haruki Mizoguchi, Akira Sakakura*
J. Org. Chem. 2020, 85 (2), 798–805.
DOI: 10.1021/acs.joc.9b02811
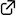

A formal total synthesis of manzacidin B is described. β,β-Disubstituted γ-hydroxy-β-aminoalcohol, the key structure of manzacidin B, is stereoselectively constructed via sequential Henry reactions. By taking advantage of noncovalent interactions, such as intramolecular hydrogen bonding and chelation, we could diastereodivergently control the stereoselectivity of the Henry reaction.
(2019.12.18)
Publication
"Toward the Synthesis of SB-203207: Construction of Four Contiguous Nitrogen-Containing Stereogenic Centers"
Ichiro Hayakawa,* Anna Nagayasu, Akira Sakakura*
J. Org. Chem. 2019, 84 (23), 15614–15623.
DOI: 10.1021/acs.joc.9b02627 
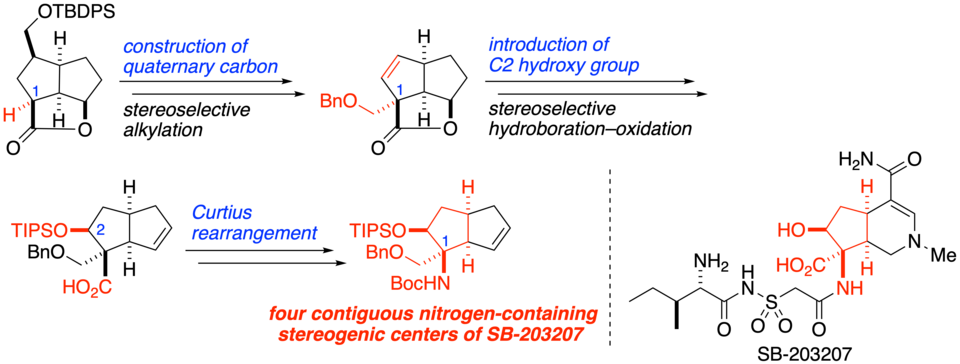
SB-203207 is an altemicidin-type alkaloid that potently inhibits isoleucyl tRNA synthetase activity. Its main structural feature is a hexahydro-6-azaindene framework containing a unique β-hydroxy α,α-disubstituted α-amino acid moiety on the cyclopentane portion. Herein we have established a method for constructing the four contiguous nitrogen-containing stereogenic centers of SB-203207 by using as key steps the stereoselective alkylation of bowl-shaped tricyclic lactone to construct quaternary carbon at C1, the stereoselective hydroboration–oxidation reaction to install the C2 hydroxy group, and the Curtius rearrangement to introduce a nitrogen atom onto the C1 quaternary carbon.
(2019.11.8)
Poster Presentation
"Formal Total Synthesis of Manzacidin B Based on Diastereoselective Henry Reaction"
Yuya Araki, Natsumi Miyoshi, Kazuki Morimoto, Haruki Mizoguchi, Akira Sakakura
The 61st Symposium on the Chemistryof Natural Products, International Conference Center Hiroshima, Sept 11–13, 2019, P1-14
Publication
"Toward the Synthesis of Yuzurimine-type Alkaloids: Stereoselective Construction of the Heterocyclic Portions of Deoxyyuzurimine and Macrodaphnine"
Ichiro Hayakawa,* Ryosuke Nagatani, Masaki Ikeda, Dong-eun Yoo, Keita Saito, Hideo Kigoshi, and Akira Sakakura*
Org. Lett. 2019, 21 (16), 6337–6341.
DOI: 10.1021/acs.orglett.9b02232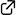
The heterocyclic portions of yuzurimine-type alkaloids, such as deoxyyuzurimine and macrodaphnine, were synthesized by using a stereoselective hydroboration−oxidation reaction to install the C20 methyl group, the intramolecular Mitsunobu reaction to construct the E-ring portion, and the intramolecular SN2 reaction to construct the F-ring portion as key steps.
(2019.7.30)
"Toward the Synthesis of Yuzurimine-type Alkaloids: Stereoselective Construction of the Heterocyclic Portions of Deoxyyuzurimine and Macrodaphnine"
Ichiro Hayakawa,* Ryosuke Nagatani, Masaki Ikeda, Dong-eun Yoo, Keita Saito, Hideo Kigoshi, and Akira Sakakura*
Org. Lett. 2019, 21 (16), 6337–6341.
DOI: 10.1021/acs.orglett.9b02232

The heterocyclic portions of yuzurimine-type alkaloids, such as deoxyyuzurimine and macrodaphnine, were synthesized by using a stereoselective hydroboration−oxidation reaction to install the C20 methyl group, the intramolecular Mitsunobu reaction to construct the E-ring portion, and the intramolecular SN2 reaction to construct the F-ring portion as key steps.
(2019.7.30)
Publication
"Enantioselective 1,3-Dipolar Cycloaddition Reaction of Nitrones with α-(Acyloxy)acroleins Catalyzed by Dipeptide-Derived Chiral Tri- or Diammonium Salts"
Chihiro Kidou, Haruki Mizoguchi, Tatsuo Nehira, Akira Sakakura*
Synlett 2019, 30 (15), 1835–1839.
DOI: 10.1055/s-0039-1690133
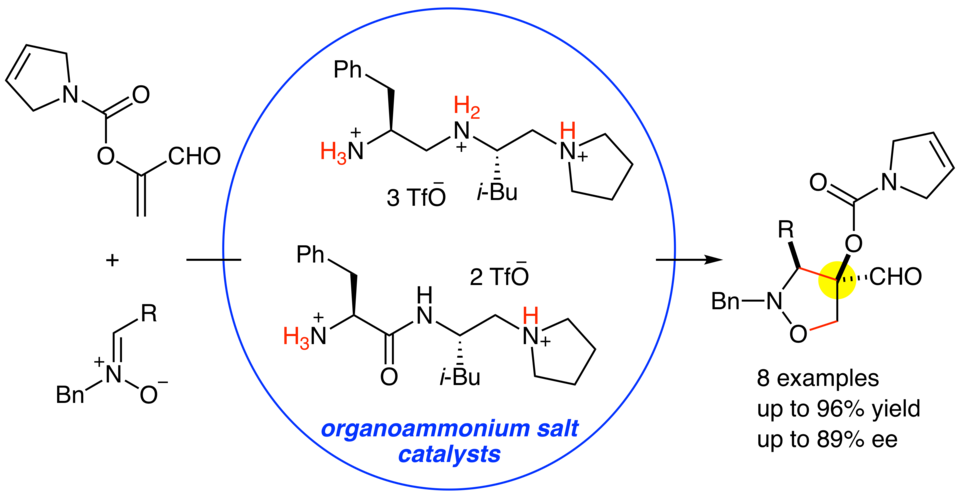
Organoammonium salts of dipeptide-derived chiral triamines or diamines with TfOH catalyzed the enantioselective 1,3-dipolar cycloaddition reactions of α-acyloxyacroleins with nitrones to give the corresponding adducts in good yields (up to 96%) and with high diastereo- and enantioselectivities (up to 89% ee). Although α-(p-methoxybenzoyloxy)acrolein is rather unstable under the reaction conditions, α-(3-pyrroline-1-carbonyloxy)acrolein is stable enough to be smoothly converted into the corresponding adducts with the aid of the chiral organoammonium salt catalysts.
(2019.7.30)
"Enantioselective 1,3-Dipolar Cycloaddition Reaction of Nitrones with α-(Acyloxy)acroleins Catalyzed by Dipeptide-Derived Chiral Tri- or Diammonium Salts"
Chihiro Kidou, Haruki Mizoguchi, Tatsuo Nehira, Akira Sakakura*
Synlett 2019, 30 (15), 1835–1839.
DOI: 10.1055/s-0039-1690133
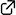

Organoammonium salts of dipeptide-derived chiral triamines or diamines with TfOH catalyzed the enantioselective 1,3-dipolar cycloaddition reactions of α-acyloxyacroleins with nitrones to give the corresponding adducts in good yields (up to 96%) and with high diastereo- and enantioselectivities (up to 89% ee). Although α-(p-methoxybenzoyloxy)acrolein is rather unstable under the reaction conditions, α-(3-pyrroline-1-carbonyloxy)acrolein is stable enough to be smoothly converted into the corresponding adducts with the aid of the chiral organoammonium salt catalysts.
(2019.7.30)

