Fishery Products
Cortistatins, anti-angiogenic compounds from the Indonesian marine sponge Cortisium simplex
Cortistatins A-L are a series of unprecedented isoquinolynyl steroids isolated from the Indonesian marine sponge, Cortisium simplex, by Kobayashi et al. (Osaka Univ., Japan). These compound are expected to be good leads of anti-cancer agent, since these showed selective growth inhibition against HUVEC.
Analogs of cortistatins were synthesized from estrone by using the Suzuki-Miyaura coupling reaction as the key step. The estrone-isoquinoline hybridized compound showed selective inhibitory activity against the proliferation and VEGF-induced migration of HUVEC. Studies on their total synthesis are under way from Hajos-Parrish ketone.

Co-worker: Dr. Takeo Usui (Tsukuba Univ., Japan), Dr. Hiroyuki Osada & Dr. Tamio Saito (RIKEN, Japan)
128. "Synthesis and Anti-angiogenic Activity of Cortistatin Analogs"
Yuuki Sato, Hiroshi Kamiyama, Takeo Usui, Tamio Saito, Hiroyuki Osada, Shigefumi Kuwahara, Hiromasa Kiyota, Biosci. Biotechnol. Biochem., 72(11), 2292-2297 (2008).
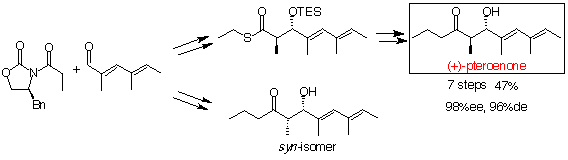
Pteroenone, antifeedant of "Sea Butterfly"
The pteropod Clione antarctica (Clione limacina) is a shell-less, pelagic mollusc which blooms each austral summer in McMurdo Sound, Antarctica. There has been known the curious relationship between C. antarctica and an antarctic hyperiid amphipod, Hyperiella dilatata, which is a prey of several antarctic fishes. Predatory fishes do not eat the amphipod grasping a pteropod on its dorsum, due to a feeding deterrent produced by the pteropod. Yoshida et al. searched for this defensive chemical substance, and isolated a compound named pteroenone. The first total synthesis of (+)-1 and its 3 diastereoisomers was achieved.
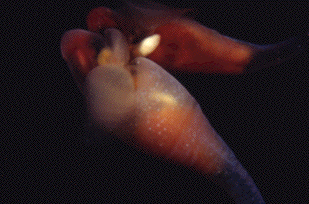

by Prof. Baker
Co-worker: Dr. Bill J. Baker (South Florida Univ.)
147. "Synthesis and Fish Antifeedant Activity of Pteroenone and its Diastereomers, a Defensive Metabolite of the Abducted Antarctic Pteropod Clione antarctica"
Hiroki Asao, Yoko Nakamura, Yukito Furuya, Tsutomu Hasaba, S. Kuwahara, B. J. Baker, H. Kiyota, Helv. Chim. Acta, 93 (10), 1933-1944 (2010).
71. "First Synthesis of (+)-Pteroenone, a Defensive Metabolite Isolated from Clione antarctica"
Yoko Nakamura, H. Kiyota, B. J. Baker, S. Kuwahara, Synlett, 635-636 (2005).
Didemniserinolipid B (Synthesis and structure revision) (at Cambridge Univ., UK)
A synthetic study of two possible diastereomers of (+)-didemniserinolipid B, the first natural serinolipid isolated from a tunicate Didemnum sp., revealed that the natural product corresponds to a 31-sulfate. The absolute configuration of 1 was also determined to be 8R,9R,10R,13S,30S. We have also developed a microwave-assisted sulfation of unreactive hydroxyl groups, for the introduction of the sulfate at the C-31 position in the natural product.
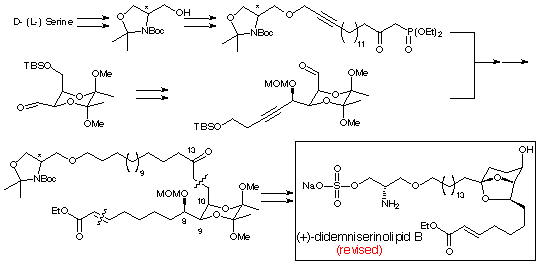
53. "Synthesis, Structure Revision and Absolute Configuration of (+)-Didemniserinolipid B, a Serinol Marine Natural Product from a Tunicate Didemnum sp."
H. Kiyota, D. J. Dixon, C. K. Luscombe, S. Hettstedt, S. V. Ley, Org. Lett., 4, 3223-3226 (2002).
94. "Synthesis of Marine Natural Products with Bicyclic and/or Spirocyclic Acetals" REVIEW
H. Kiyota, in "Topic in Heterocyclic Chemistry 05: Marine Natural Products", ed. H. Kiyota, Chapter 4, Springer 2006, pp 65-96.
Dendryphiellin C (at the Univ. of Tokyo)
Total synthesis of dendryphiellin C, isolated from a marine fungus, Dendryphiella sarina was achieved.
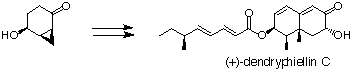
20. "Synthesis of dendryphiellin C, a trinor-sesquiterpene from a marine source"
H. Akao, H. Kiyota, T. Nakajima, T. Kitahara, Tetrahedron, 55, 7757-7770 (1999).




